
As a stream of charged electrons, a cathode ray has no color. What color does a cathode ray have? 2 marksĪns. Cathode rays can be deflected by electric and magnetic fields. What are the two ways that Cathode Rays can be Deflected? 2 marksĪns. He found out that the cathode rays were charged negatively. Electron was found by J.J Thomson through the cathode ray tube. This is because cesium readily releases electrons when it is hit by the light or is heated. Cesium is used in the formation of cathode-ray tubes. Which material is used to make cathode ray tubes and why? 2 marksĪns. They are negatively charged and are blocked by electric and magnetic forces. They're strong and capable of completing the task.

They travel in a straight line and cast long shadows. Cathode rays develop in an evacuated tube and travel toward the anode via the negative electrode or cathode. What characteristics do cathode rays have? 2 marksĪns. The Cathode Ray Experiment was performed by J.J Thomson which led to the discovery of electrons.They can pierce thin aluminum plates and have the ability to make phosphors glow.The electrodes and gas present in the cathode ray tube do not affect their properties and gas can be ionized by cathode rays.When cathode rays are impeded by obstacles they produce a shadow and normally move in straight lines.Cathode Rays are rays that flow from the negative end to the positive end of an electrode in a vacuum.CRT screens are also used in oscilloscopes, which are machines that measure and display voltages.Ĭathode Ray Oscilloscope Things to remember.Black and white televisions and ancient computer terminals are examples of monochromatic CRTs.Three electron cannons correlate to corresponding types of phosphors in color CRTs, one for each main color (red, green, and blue). Televisions and computer monitors used to use CRT technology.A vacuum tube holding an electron cannon and a screen lined with phosphors are the main components of a cathode ray tube (CRT).They also discovered that the quantity of voltage, kind of gas, and constituent ratios did not affect the natural, physical, and behavioral features of electrons. The theory helped future physicists in understanding the modern structure of an atom. Thomson derived a conclusion after the experiment that the rays that moved from the cation to the anion were negative. The phosphorescent substance was put in such a way that when the rays strike it, little sparks of light appear, allowing the stream of rays to be detected.The cathode rays begin deflecting and repelling from the dipole and moving towards the anode as a result.A dipole is used to detect and measure the beam that was created.A closed-loop circuit propagates electric current.Ionization of partial air inside the terminal due to high voltage causes gas to become the conductor.The equipment is set up so that the terminals have a high voltage and the internal pressure is decreased by eliminating air from the tube.The cathode ray was redirected away from the negatively charged plate and towards the positively charged plate. Thomson used two opposingly charged electric plates around the cathode ray to test the particles' characteristics. Thomson, a scientist, began working with cathode ray tubes in the late 1800s.
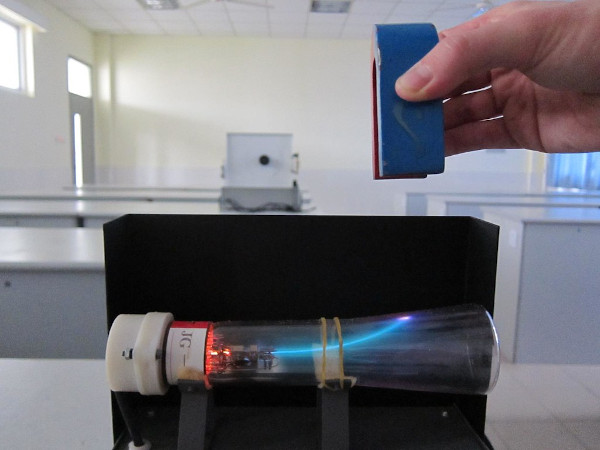
Cathode rays are not visible, but they were first identified in early vacuum tubes when they impacted the tube's glass wall, energizing the atoms and causing them to emit light-a glow known as fluorescence.In simple words, rays traveling from the negative end to the positive end of an electrode within a vacuum are known as Cathode Rays.A focused beam of electrons is deflected by magnetic or electric fields in cathode ray tubes, in order to produce the image in a traditional television set (CRTs).Ĭathode Rays used to produce images in traditional Television The constituents of cathode rays were the first to be found. When a voltage is given to an evacuated glass tube with two electrodes, electrons emitted from the cathode cause the glass opposite the negative electrode to glow. In vacuum tubes, cathode rays (electron beams or e-beams) are electron streams.When the electron beam hits the phosphor-coated screen, it creates a small, brilliant visible spot on the fluorescent screen.

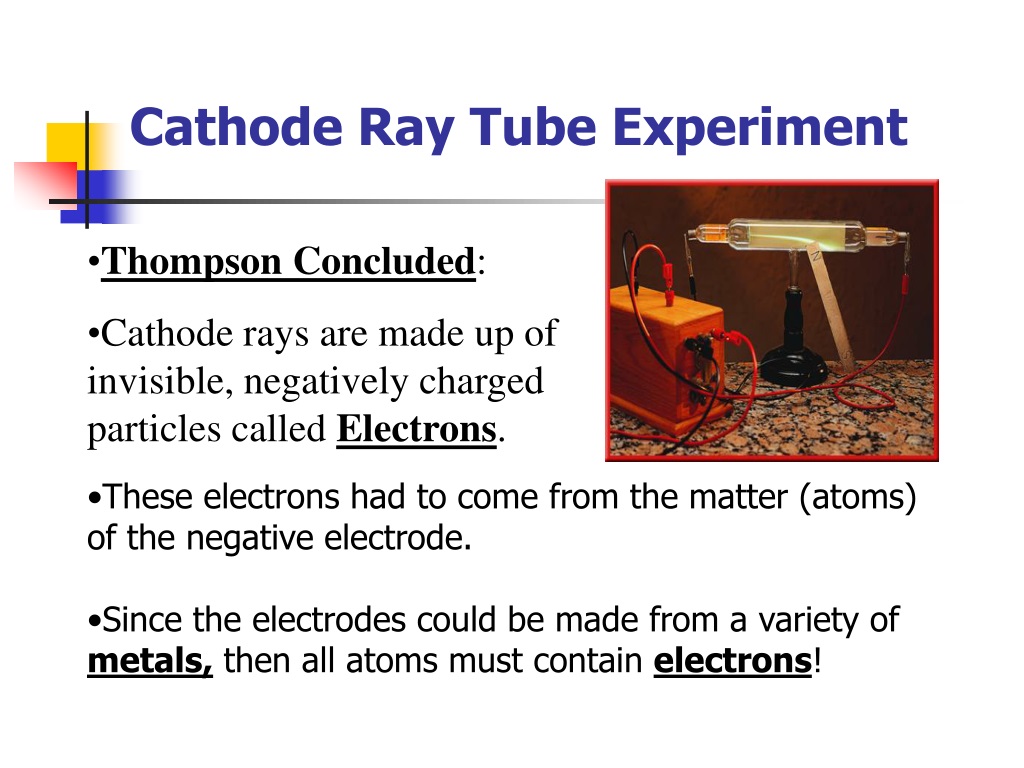
CRTs are used in the majority of desktop computer displays.Īs seen in the image, a cathode ray tube is made up of numerous fundamental components: A cathode ray tube(CRT) is a kind of vacuum tube producing images when an electron beam collides with a phosphorescent surface.


 0 kommentar(er)
0 kommentar(er)
